FDA Approves Historic RSV Vaccine for Pregnant Women, Promising Protection Against Deadly Lung Infection for Newborns
By: WE Staff | Tuesday, 29 August 2023
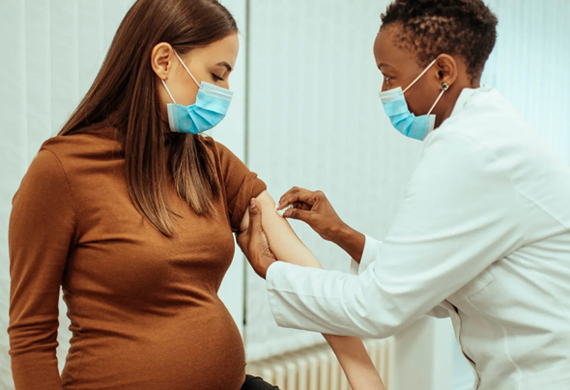
The first-ever respiratory syncytial virus (RSV) immunisation made specifically for pregnant women has been approved by the American food and drug administration, marking a historic accomplishment. A revolutionary vaccine called Abrysvo promises to safeguard foetuses and provide essential protection against the fatal lung infection from birth onward.
The respiratory syncytial virus, or RSV, causes newborns to wheeze during the autumn and winter, flooding hospitals. In this endeavour, the large pharmaceutical corporation Pfizer has emerged as a pioneer, and their recently authorised vaccine is anticipated to revolutionise early-life immunity. The immunisation will provide protection against an infant's susceptibility to severe RSV infections, which lasts from birth through the first six months of life.
The Centres for Disease Control and Prevention are now responsible for formulating guidelines for the use of Abrysvo during pregnancy. Dr. Elizabeth Schlaudecker of Cincinnati Children's Hospital in Ohio, who took part in Pfizer's worldwide vaccination study, commended maternal immunisation as a revolutionary approach to safeguarding children. Vaccinations, according to Dr. Schlaudecker, might make a significant impact in the upcoming RSV season provided they were administered correctly the first time.
RSV is a major risk to infants while being a normally mild infection in healthy individuals. When a baby contracts the virus, their airways swell, which can lead to respiratory discomfort and even pneumonia. In the US, RSV hospitalises 58,000 to 80,000 children under the age of five every year and results in several hundred fatalities.
The significance of maternal protection is highlighted by the fact that infants are born with a compromised immune system and heavily rely on their mothers for disease resistance during the first few months of life. Towards the conclusion of pregnancy, a single injection is administered during the immunisation process, providing the mother plenty of time to build antibodies. These protective compounds are given to the foetus, where they remain until they are activated at birth.
This tactic is comparable to the regular immunisation of expectant mothers against a variety of diseases, including the flu, whooping cough, and more recently, COVID-19.
Although Pfizer's study, which involved roughly 7,400 pregnant women and their kids, indicated that the maternal vaccination did not always protect against mild RSV infection, it did demonstrate a surprising 82 percent efficacy in preventing severe infections in the first three months following birth. For the first six months of the protection, 69% of major illnesses may be prevented.
The predominant adverse reactions to the immunisation were fatigue and regional discomfort at the injection site. In particular, the FDA recommended vaccination between weeks 32 and 36 of pregnancy to lower the little risk of an early birth, which was observed in a very small percentage of mothers who got the vaccine in the experiment.
Pfizer believes that up to 20,000 newborn hospitalisations and 320,000 doctor visits may be avoided annually if a large percentage of expecting moms opt inoculation.
An alternate strategy for providing RSV protection to infants is the administration of lab-made antibodies. The U.S. Food and Drug Administration has approved Beyfortus, a new drug designed especially for infants less than 8 months. The release of this single-dose drug, which is made by Sanofi and AstraZeneca, is anticipated for the autumn of 2020.
Doctors, notably those from Cincinnati Children's Hospital, seek to combine these cutting-edge treatments to optimise neonatal protection against RSV. While actively participating in Pfizer's immunisation study while pregnant, Dr. Maria Deza Leon shared her personal experience and stressed the importance of RSV infections in babies.
Because her son Joaquin was born a month after she had the immunisation, allaying parents' concerns about their children's health, Dr. Deza Leon is still upbeat about the future.
Most Viewed
- 1 Women's Health Startup HerMD Closing Doors Amid Industry Challenges
- 2 5 Famous Women in Indian Armed Forces
- 3 Saudi Women No longer Require Male Permission for Clothing Choices, says Prince MbS
- 4 Kolkata Medtech Startup Innovodigm Raises Rs 5.5 Crore Seed Funding Led by IAN Group
- 5 Yamunanagar's Kashish Kalra Honoured after Securing 111th Rank in UPSC Civil Services Exam
- 6 Madurai Appoints Its First Woman Corporation Head
- 7 IAS Vijayalakshmi Bidari Appointed as the new Nagpur Divisional Commissioner
- 8 American Entrepreneur Lucy Guo Overtakes T Swift to become Youngest Female Billionaire
- 9 ICC Women's World Cup 2025 Trophy Showcased at Indore's Holkar Stadium
- 10 Aparna Saxena's Beauty Venture AntiNorm Launches in India
- 11 Vidya Nataraj Co-Founded BlueStone Jewellery & Lifestyle files IPO
- 12 5 Women Freedom Fighters of India
- 13 Dr. G Krishnapriya appointed as CEO for Trichy
- 14 M3M & Sirona Partner to Introduce Menstrual Hygiene Vending Machines in 15 Locations
- 15 Punjab Govt launches SHE Cohort 3.0 Supporting Tech-led Women Startups
- 16 Indian origin Lawyer, Sweena Pannu appointed as the US New Superior Court Judge
- 17 The Aurora Tech Award recognizes 4 Indian Women-led Startups
- 18 Kerala's Republic Day parade featured an all-female tableau
- 19 Manisha Kabbur Becomes Karnataka's First Woman International Karate Coach
- 20 Director K. S. Ravikumar's Daughter Maalica Ravikumar Launches Life Coaching Company 'Evergrowth Academy' for Women
- 21 Leezu's Raises Pre-Seed Funding to Accelerate Growth in Sexual Wellness Industry
- 22 Sattu: Super-easy summer drink for PCOS gut healing
- 23 Swathi Nelabhatla creates Sitha App, India's First Women-Exclusive Gig Platform
- 24 7 Timeless Female Kathak Dancers & their Iconic Legacies
- 25 Meet 7 Iconic Women Architects of Modern India & their Most Impactful Work
- 26 This Woman-led Insuretech Startup is Helping Bridge the Education Financing Gap in India
- 27 Women Leaders Share Lessons Learnt from India Women's WC Win
- 28 5 Enterprising Women Founders Powering Singapore's Tech & Innovation Landscape
- 29 4 Women. 4 Stories. One Vision for Smarter, Stronger Healthcare
- 30 Global Gender Gap Narrows to 68.8%, But Full Equality 123 Years Away: WEF Report 2025
- 31 Changemakers: 7 Women Entrepreneurs Taking the Make in India Movement Forward
- 32 Meet Lucy Guo, The Youngest Self-Made Female Billionaire Disrupting Tech
- 33 How Women are Driving India's Festive Online Shopping Surge






